29+ Why Are Noble Gasses Unreactive
Xenon is a noble gas and has similar. Web 0000 - Are noble gases largely unreactive0040 - Which is the most unreactive gas0108 - What are the uses of noble gas0141 - Why is Krypton so unreacti.

Difference Between Inert Gases And Noble Gases Definition Properties Examples
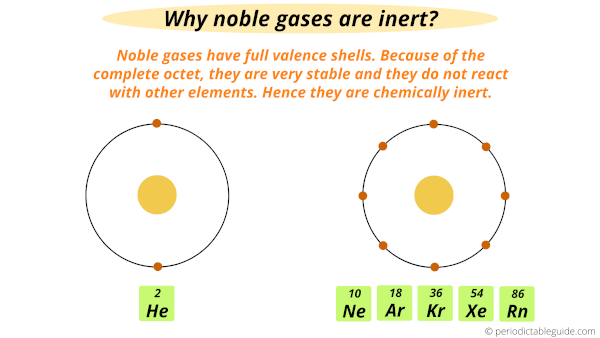
Web Noble gas atoms are unreactive because they have a fully filled valence shell eg.

. On the outer shell of the noble gasses there is no electrons to be shared because it is full. Web The noble gasses are unreactive because of the elctronic structure. Web Noble gases are six monoatomic gaseous elements found in nature that share similar chemical properties.
Web Noble gases have stable configuration due to the presence of 8 electrons in their valence shell ie they are unreactive. Web New understanding of noble gas behavior may solve a long-standing mystery surrounding the atmospheric abundance of xenon. This means they have completely filled s and p sublevels which gives.
Web Best Answer. They have 8 valence electrons meaning that they are happy and stable. Have an atomicity of one.
Web Noble gases contain a full valence of electrons. These grades are the stepping stone to your future. Web The noble gases dont react because they have their full orbital.
They do not need to interact with other atoms in order to get stable. They dont need any more. Web I want to help you achieve the grades you and I know you are capable of.
Web The noble gases are unreactive because they. Have same number of electrons. Web Why are noble gases relatively unreactive.
This makes noble gases unreactive. The reason as to why these elements are called noble is because. Because of this configuration they are i difficult to reduce electrons must enter the next valence shell and ii difficult to oxidize.
Web However noble gases already have a complete octet. Other elements react by gaining or losing electrons to. They have a completely filled outer level.
-The elements present in. Web Due to their stable nature noble gases are highly unreactive and do not react with any other atom or molecule to give chemical reaction. One of the defining properties of this group is the unreactivity of these elements.
Since they are already totally stable on their own it takes too much energy. Even if you dont want to stud. The Noble gases have a completely filled valence electron shell.
Are gases with low density. Web The elements that make up the last group in the periodic table are called noble gases. He is duplet configuration Ne is octet configuration hence they do not readily bond with other.

Noble Gas Definition Elements Properties Characteristics Facts Britannica

Inert Gas Overview Types Examples What Are Noble Gases Video Lesson Transcript Study Com

Group 0 Noble Gases Shalom Education

Noble Gases Trends And Patterns Scienceaid

Why Don T Noble Gases Bond Video Lesson Transcript Study Com
Igcse Chemistry 2017 1 24 Understand Why The Noble Gases Group 0 Do Not Readily React
Xenon And Other Noble Gas Compounds Gesellschaft Deutscher Chemiker E V
Periodic Trends Inert Gas Radii W3schools
Where Are Noble Gases Located On The Periodic Table
Noble Gas Definition Elements Facts And Properties
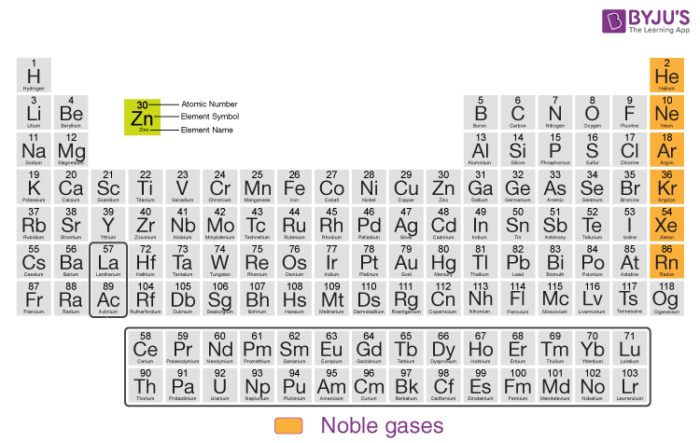
Uses Of The Noble Gases Noble Gas Elements Uses Applications Videos And Faqs Of Noble Gases
Synthesis Of Noble Gas Compounds Defying The Common Perception Springerlink
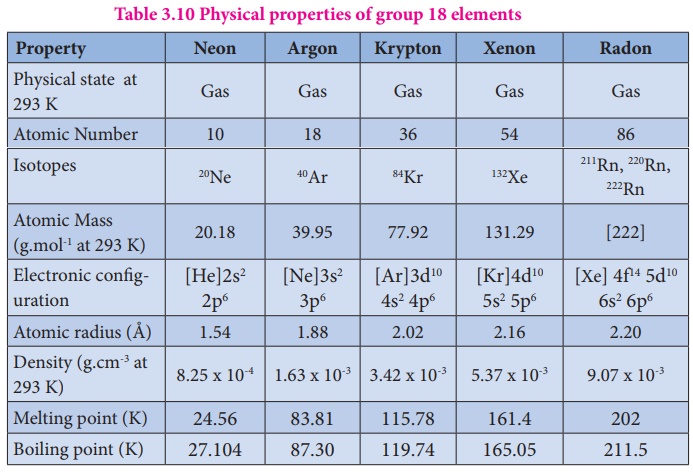
Group 18 Inert Gases Elements Occurrence Preparation Properties Structure Uses

Noble Gas Wikipedia
:max_bytes(150000):strip_icc()/Xenonhexafluoride-56a12d265f9b58b7d0bccc78.png)
Noble Gas Chemical Compounds
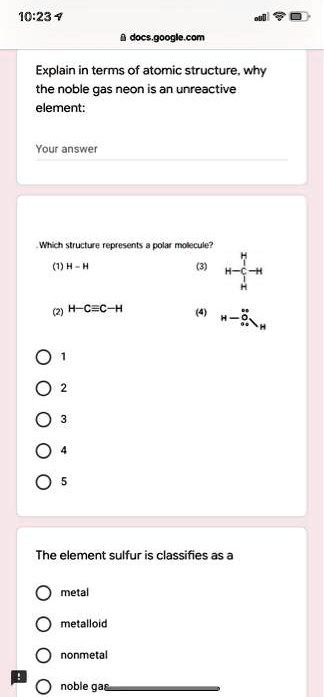
Solved 10 23 Docs Google Com Explain In Terms Of Atomic Structure Why The Noble Gas Neon Is An Unreactive Element Your Answer Witc Structure Represents Pola Motecule H 2 H C C H 4 3
How Do Noble And Inert Gases Differ Quora